ELECTROREDUCTION OF NITROUS OXIDE WITH NANOSTRUCTURED SNO2 MATERIALS
NANOSCIENCE

Lab: LCC
Duration: NanoX master Internship (8 months part-time in-lab immersion)
Latest starting date: 06/10/2025
Localisation: Molecules and Composites for Optics (LCC) - Nanostructures and Organometallic Chemistry (LPCNO)
Laboratoire de Chimie de Coordination du CNRS (LCC-CNRS)
205, route de Narbonne
31400 TOULOUSE - FRANCE
Supervisors:
Nuria ROMERO nuria.romero@lcc-toulouse.fr
Nicolas QUERYIAUX nicolas.queyriaux@lcc-toulouse.fr
Work package:
Nitrous oxide is an important atmospheric pollutant emitted by human activities, acting both as the third most important greenhouse gas and the main ozone-depleting agent. Its tropospheric concentration is constantly rising when compared to pre-industrial levels, driven by anthropogenic perturbations of the nitrogen cycle: large-scale use of synthetic fertilizers in modern agriculture, chemical-processing manufactures, fossil fuels burning…[1] Thermal decomposition, direct catalytic decomposition and selective catalytic reduction of N2O have emerged as interesting strategies to mitigate N2O emissions and decrease its environmental footprints.[2] However, they typically suffer from energy-intensive processes and are characterized by a high level of waste production. An alternative option lies in the utilization of electrocatalytic N2O decomposition, which reduces nitrous oxide at an electrode surface. Such approach displays significant advantages, including room-temperature reaction, low energy consumption and absence of external reducing agents.
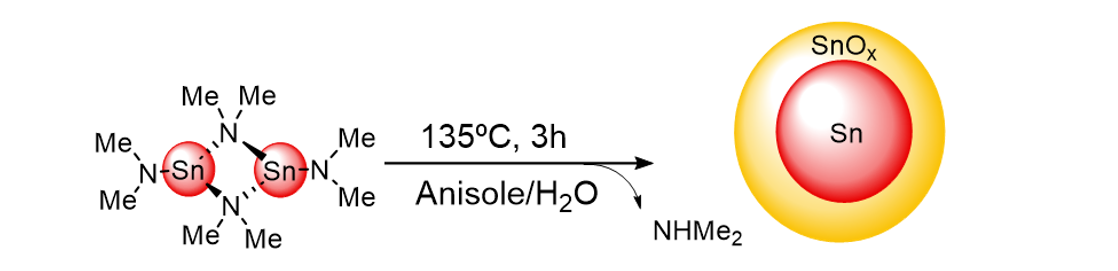
Beyond the typical noble metal-based electrodes (Pt, Pd),[3] alternative electroactive materials relying on abundant metal oxides have been explored. Bulk Tin(IV) oxide (SnO2), in particular, have shown promising catalytic properties when employed in aqueous media: faradaic yields of ca. 75% for N2O reduction and high selectivity against proton reduction.[4] Nanostructuration of SnO2 is therefore a promising pathway to obtain highly active and selective electrocatalysts. Small and well dispersed core-shell Sn@SnO2 nanoparticles can be obtained according to Scheme 1.
In this internship, investigation of such materials towards N2O electroreduction will be conducted. Effects of doping, size and surface tuning, support nature (carbonaceous vs conducting glass) and experimental conditions (pH, substrate feed…) will systematically be examined to gain insight into the main parameters driving catalysis in this family of materials.
References:
References:
[1] V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, Ö. Yelekçi, R. Yu, B. Zhou, Eds. , Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2021.
[2] X. Wu, J. Du, Y. Gao, H. Wang, C. Zhang, R. Zhang, H. He, G. (Max) Lu, Z. Wu, Chem. Soc. Rev. 2024, 53, 8379–8423.
[3] A. Kudo, A. Mine, Journal of Electroanalytical Chemistry 1996, 408, 267–269.
[4] A. Kudo, A. Mine, Applied Surface Science 1997, 121–122, 538–542.
Areas of expertise:
Nanoparticles, electrochemistry
Required skills for the internship:
The internship will be co-supervised by Dr. N. Queyriaux and Dr. Nuria Romero. Solid skills in nanoparticles synthesis and characterization (TEM/HRTEM, IR, XRD, XPS, ICP, NMR, etc.), electrode assembly and electrocatalysis will be acquired.
To apply, please attach a CV, M1 results and the most recent internship report to your application.
