Interfacial ionic liquid-based nanocatalysts for low temperature CO2 reduction
NANOSCIENCE

Lab: LPCNO
Duration: NanoX master Internship (8 months part-time in-lab immersion)
Latest starting date: 15/10/2022
Localisation: LPCNO and LCC
Supervisors:
Katerina Soulantica ksoulant@insa-toulouse.fr
Philippe Serp philippe.serp@ensiacet.fr
This research master's degree project could be followed by a PhD
Work package:
Efficient CO2 conversion using green H2 from renewable sources can contribute to the reduction of CO2 emissions and limit global warming.[1] Useful building blocks, such as CH4, CH3OH, CO or HCOOH can be obtained from the catalytic thermal reduction of CO2. Nickel a non-noble and abundant metal is particularly attractive for this reaction, and supported Ni catalysts generally lead to the production of methane but with moderate activity.[2]
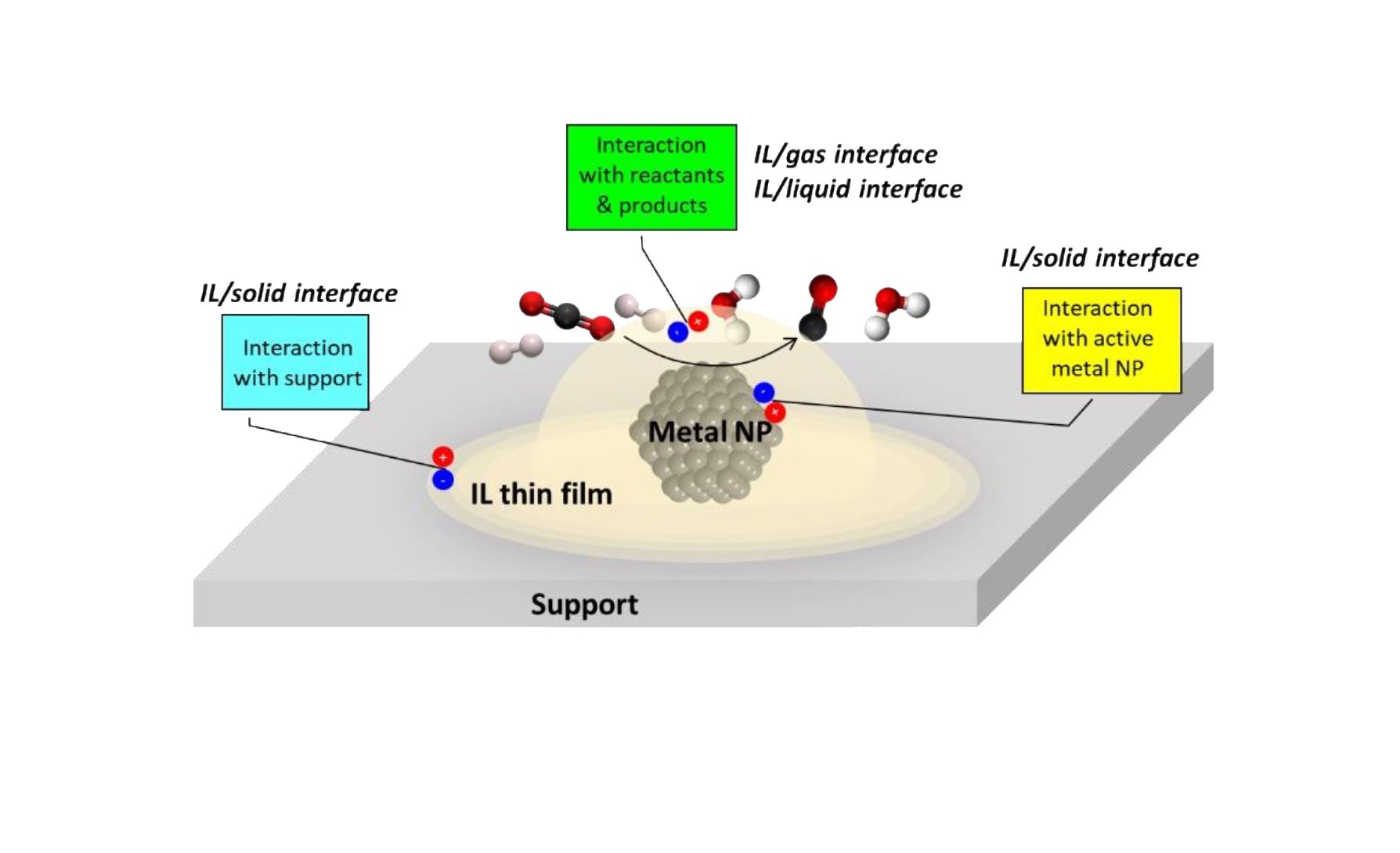
A way to regulate catalyst activity but also selectivity can be to create an interface between supported metal nanoparticles (MNPs) and a specific medium with adjustable properties, such as ionic liquids (ILs), which would allow controlling some of the key reaction parameters (metal dispersion, electron density, metal-support interaction). Indeed, ultrathin layers of ILs supported on porous supports either exhibit high intrinsic catalytic activity, or serve as peculiar solvents and stabilizers for an introduced active catalyst.[3]
In this context, the project aims at developing a new generation of innovative supported catalysts for CO2 hydrogenation combining supported MNPs and a task-specific IL, in order to achieve an interfacial ionic liquid catalysis for low temperature thermal CO2 reduction.
In LPCNO, the student will produce by an organometallic route shape and size controlled Ni nanoparticles stabilized by a selected IL. Such ligand-protected MNPs will then be immobilized on TiO2 by liquid phase impregnation. Ultrathin layers of IL will finally be deposited on the catalysts by liquid phase procedures. All catalysts will be fully characterized by conventional techniques (ICP, N2 adsorption, XRD, chemisorption, TEM, STEM-HAADF, XPS, VSM) at different level of preparation (before and after IL deposition).
The student will also evaluate the catalytic performance of this catalyst for CO2 hydrogenation in LCC and compare it with the one of a conventionally prepared Ni/TiO2 catalyst.[4]
References:
[1] S. C. Peter (2018). Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 3: 1557-1561. 10.1021/acsenergylett.8b00878
[2] W. K. Fan & M. Tahir (2021). Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 9: 105460. 10.1016/j.jece.2021.105460
[3] B. V. Romanovsky & I. G. Tarkhanova (2017). Supported ionic liquids in catalysis. Russ. Chem. Rev. 86: 444-458. 10.1070/rcr4666
[4] D. Messou, V. Bernardin, F. Meunier, M. B. Ordoño, A. Urakawa, B. F. Machado, V. Collière, R. Philippe, P. Serp & C. Le Berre (2021). Origin of the synergistic effect between TiO2 crystalline phases in the Ni/TiO2-catalyzed CO2 methanation reaction. J. Catal. 398: 14-28. 10.1016/j.jcat.2021.04.004
Areas of expertise:
CO2 utilization; metal nanoparticles; ionic liquids; catalysis
Required skills for the internship:
The candidate must have folwed courses in inorganic chemistry. A basic knowledge of metal particle characterization techniques is desirable. A basic experience in nanoparticle synthesis and in catalysis will be welcome.
