POLYMERS SOFT TEMPLATES FOR SYNTHESIS OF CATALYTIC MATERIALS
CHEMISTRY & GREEN CHEMISTRY

Lab: IMRCP
Duration: NanoX master Internship (8 months part-time in-lab immersion)
Latest starting date: 02/01/2025
Localisation: Laboratory: Chemistry of colloids, polymers & complex assemblies
Softmat – UMR 5623
Université Toulouse III-Paul Sabatier
Bâtiment 2R1
118 route de Narbonne
30062 Toulouse - FRANCE
Supervisors:
Barbara LONETTI barbara.lonetti@univ-tlse3.fr
Diana CIUCULESCU-PRADINES eliza.ciuculescu-pradines@univ-tlse3.fr
Work package:
Block copolymers (BCPs) spontaneously assemble in solution leading to polymer aggregates
with different ordered nanostructures, such as vesicles, cubosomes and hexosomes. These assemblies
are highly useful as “soft” templates for the synthesis of various functional materials [1,2] One interesting
benefit is the possibility to easily remove the template therefore leading to materials with high porosity and
high specific surface area.
We have already reported on a simple method to obtain controlled three-dimensional hybrid
architectures, formed by gold nanoparticles and a double hydrophilic block copolymer, specifically the
poly(acrylic acid)-block-poly(N-vinyl-2-pyrrolidone) (PAA-b-PVP), directly in aqueous medium. The same
copolymer has been used for template-mediated synthesis of ZrO2.
In order to reinforce and modulate the structure of these assemblies, the incorporation of naturally
occurring polyphenols is an interesting option. [3,4] Indeed, the presence of catechol and gallol groups
enable the interaction of polyphenols with different substrates through hydrogen bonding, metal
coordination, hydrophobic and -interactions. Additionally, they are also able to reduce noble metal ions
(Au3+, Pt2+, Ag+) and to coordinate to the surface of the formed nanoparticles.
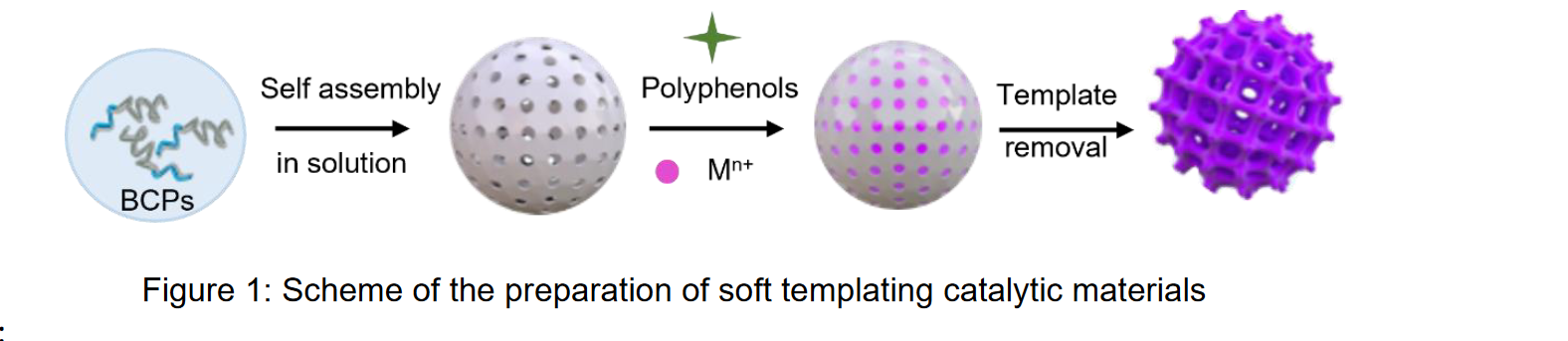
During this internship the objectives will be: i). to prepare block copolymers assemblies in the
presence of polyphenols and different metallic ions (Au3+, Ag+, Zr4+, Fe3+); ii) convert them into
corresponding metal or metal oxide derivatives (Figure 1) iii) characterize the assemblies (scattering
techniques, electronic microscopy, IR and UV-Vis measurements, etc.) iv) evaluate the catalytic activity of the best candidate.
References:
[1] C. Li, Q. Li, Y.V. Kaneti, D. Hou, Y. Yamauchi, Y. Mai, Self-assembly of block copolymers towards
mesoporous materials for energy storage and conversion systems, Chem Soc Rev 49 (2020) 4681–4736.
https://doi.org/10.1039/D0CS00021C.
[2] W. Xie, X. Huang, C. Zhu, F. Jiang, Y. Deng, B. Yu, L. Wu, Q. Yue, Y. Deng, A Versatile Synthesis Platform
Based on Polymer Cubosomes for a Library of Highly Ordered Nanoporous Metal Oxides Particles, Adv. Mater. 36
(2024) 2313920. https://doi.org/10.1002/adma.202313920.
[3] J. Zhou, Z. Lin, Y. Ju, Md.A. Rahim, J.J. Richardson, F. Caruso, Polyphenol-Mediated Assembly for Particle
Engineering, Acc. Chem. Res. 53 (2020) 1269–1278. https://doi.org/10.1021/acs.accounts.0c00150.
[4] H. Zhang, M. Zhang, R. Liu, T. He, L. Xiang, X. Wu, Z. Piao, Y. Jia, C. Zhang, H. Li, F. Xu, G. Zhou, Y. Mai,
Fe3O4-doped mesoporous carbon cathode with a plumber’s nightmare structure for high-performance Li-S batteries,
Nat. Commun. 15 (2024) 5451. https://doi.org/10.1038/s41467-024-49826-5.
Areas of expertise:
Polymer science, colloids, inorganic nanoparticles, functional materials
Required skills for the internship:
The skills required are in the field of physical chemistry and coordination chemistry.
Scientific curiosity and social skills will be appreciated.
