SUPRAMOLECULAR HETEROGENEOUS CATALYSIS
CHEMISTRY & GREEN CHEMISTRY

Lab: LCC
Duration: NanoX master Internship (8 months part-time in-lab immersion)
Latest starting date: 02/01/2025
Localisation: Laboratoire de chimie de coordination
205, route de Narbonne
31077 TOULOUSE - FRANCE
Supervisors:
M. Rosa AXET rosa.axet@lcc-toulouse.fr
Hiroya ISHIKAWA hiroya.ishikawa@lcc-toulouse.fr
Work package:
The effects of ligands coordinated to transition metals on catalysis are well documented,[1]
and rational ligand modification to control activity and selectivity of homogeneous catalysts is
also well explored. In order to increase the level of control in transition metal catalysis,
supramolecular substrate orientation has been recently introduced.[2] In such a strategy, the
substrate is oriented with respect to the active site by means of supramolecular interactions
such as hydrogen bonds. This level of selectivity is more challenging to achieve in
heterogeneous catalysis.[3] Single-atom catalysts (SACs) are emerging materials in the field of
catalysis, due to its straightforward synthesis and the possibility to modify supports to control
the stability, local environment, and electronic properties of the isolated atoms, therefore
open the door to tailor their properties as heterogeneous catalysts.[4] Modification of the
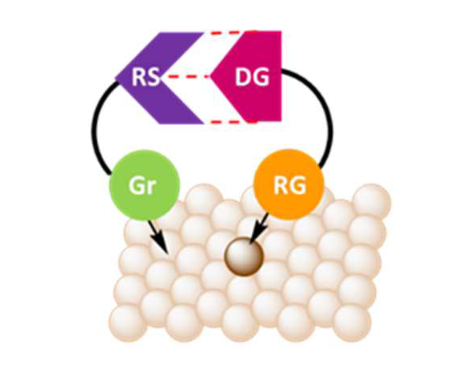
support with supramolecular strategies will allow to further predetermine the local
environment of the isolated metallic atom and pre-organization of the desired substrate. The
project aims at control the selectivity of single-atom catalysts through supramolecular
substrate-ligand interactions. This strategy is especially appealing for controlling selectivity in hydroformylation (HF) reaction
using SACs. The use of Rh-based SACs for HF has seen significant advancement in recent years.[5] This growing interest is
largely due to certain catalytic systems demonstrate activity levels comparable to those of homogeneous catalysts.
Nevertheless, these examples are scarce, and in all cases described for this kind of catalysts (except the ones using phosphorus
ligand moieties, such as porous organic polymers), the origin of selectivity, if any, is unknow. Few examples in HF reaction
catalysed by Rh-based SACs on solid supports display high selectivity. For instance, for styrene HF, l/b ratios observed for Rh-
based SACs are in the range of 0.5 to 1, the highest one to date displaying an l/b of 1.6 for Rh single atoms on carbon nitride
(C3N4).[6] In order to control selectivity, we plan to use straightforward and inexpensive organic transformations on a
carbonaceous support (ideal for this aim), to keep the advantages of heterogeneous catalysts (straightforward, scalable
synthesis), while incorporating concepts of supramolecular chemistry. The project is part of a collaboration with J. Reek
(University of Amsterdam),[7] and will count with the additional supervision of Hiroya Ishikawa.[8]
References:
(1) Gillespie, J. A.; Zuidema, E.; van Leeuwen, P. W. N. M.; Kamer, P. C. J. Phosphorus ligand effects in homogeneous catalysis and rational
catalyst design; John Wiley & Sons Ltd., 2012. DOI: 10.1002/9781118299715.ch1. (2) Dydio, P.; Reek, J. N. H. Supramolecular control of selectivity in transition-
metal catalysis through substrate preorganization. Chem. Sci. 2014, 5 (6), 2135-2145. DOI: 10.1039/c3sc53505c. (3) Lu, L.; Zou, S.; Fang, B. The critical impacts
of ligands on heterogeneous nanocatalysis: A review. ACS Catal. 2021, 11 (10), 6020-6058. DOI: 10.1021/acscatal.1c00903. (4) Kaiser, S. K.; Chen, Z.; Faust
Akl, D.; Mitchell, S.; Perez-Ramirez, J. Single-atom catalysts across the periodic table. Chem. Rev. 2020, 120 (21), 11703-11809. DOI:
10.1021/acs.chemrev.0c00576. (5) Jurado, L.; Posada-Perez, S.; Axet, M. R. Carbonylation reactions using single-atom catalysts. ChemCatChem 2024,
e202400543. DOI: 10.1002/cctc.202400543. (6) Jurado, L.; Esvan, J.; Luque-Alvarez, L. A.; Bobadilla, L. F.; Odriozola, J. A.; Posada-Perez, S.; Poater, A.; Comas-
Vives, A.; Axet, M. R. Highly dispersed Rh single atoms over graphitic carbon nitride as a robust catalyst for the hydroformylation reaction. Catal. Sci. Technol.
2023, 13 (5), 1425-1436. DOI: 10.1039/d2cy02094g. (7) Liu, B.; Wang, Y.; Huang, N.; Lan, X.; Xie, Z.; Chen, J. G.; Wang, T. Heterogeneous hydroformylation of
alkenes by Rh-based catalysts. Chem 2022, 8 (10), 2630-2658. DOI: 10.1016/j.chempr.2022.07.020. (8) Reek, P. d. J. N. H.
https://www.uva.nl/en/profile/r/e/j.n.h.reek/j.n.h.reek.html. (9) Ishikawa, H. https://orcid.org/0009-0009-3815-915X.
Areas of expertise:
Catalysis, single-atom catalysis, hydroformylation, supramolecular chemistry
Required skills for the internship:
Organometallic chemistry, heterogeneous catalysis, characterization technique
