Towards symmetric hetero-carbo-graphenes: synthetic feasibility of a thiographdiyne
MATERIAL AND SURFACE SCIENCE

Lab: LCC
Duration: NanoX master Internship (8 months part-time in-lab immersion)
6 months full-time internship
Latest starting date: 01/02/2022
Localisation: Laboratoire de Chimie de Coordination, UPR-8241, Toulouse
Supervisors:
Valérie MARAVAL, Dr valerie.maraval@lcc-toulouse.fr
Remi CHAUVIN, Pr remi.chauvin@univ-tlse3.fr
This research master's degree project could be followed by a PhD
Work package:
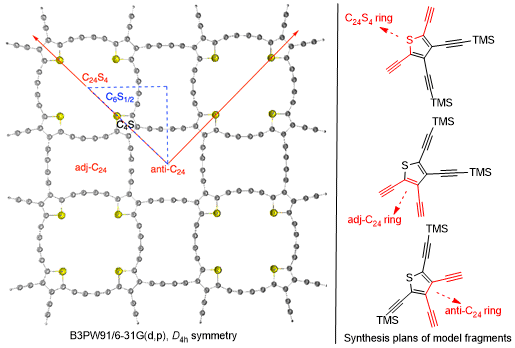
Tuning extremal properties of P6mm-symmetric graphene made of equivalent sp2-C atoms, requires introduction of chemical disorder [1]. Beyond random doping in heterographenes [2], symmetric stoichiometric "de-homogenization" preserving planar delocalization can be achieved by substitution, e.g. of C–C bonds by B–N bonds to give a hexagonal boron nitride (h-BN) layer [3], or insertion, e. g. of sp-C2 units into selected C C bonds to give graphyne and graphdiyne carbon allotropes, such as alpha-graphyne or alpha-graphdiyne [4]. The stability of these all-hexagon structures is supported by their ideal compliance to the VSEPR rule at sp2C nodes, but the higher flexibility at sp-C nodes might allow strained structures incorporating aromatic pentagons such as furan, pyrrole or thiophene rings. A symmetric "thiographdiyne" is thus devised from gamma-graphdiyne (involving 3 types of C atoms and 2 types of fundamental circuits, C6 and C18) by replacement of the C6 rings by C4S rings, thus resulting in 7 types of atoms and 4 types of fundamental circuits, C4S , C24S4 (carbo2-sulflower [5]), adj-C24 and anti-C24, the latter 4n π-e macrocycles being large enough to be devoid of antiaromatic character.
While properties of the putative material (enthalpy of formation, band structure, metal S-coordination) are studied at the periodic DFT level [6], the project aims at the synthesis of cyclic model fragments by relying on the experience of the group in carbo-mer chemistry [7].
References:
[1] G. Yang, L. Li, W. B. Lee, M. C. Ng, Sci. Tech. Adv. Mater. 2018, 19, 613.
[2] W. Zhao, C. Papp, H.-P. Steinruck, Heterographenes in Encyclopedia of Polymeric Nanomaterials, pp. 1-15, Springer, 2014.
[3] C. Jin, F. Lin, K. Suenaga, S. Iijima, Phys. Rev. Lett. 2009, 102, 195505.
[4] (a) R. H., Baughman, H. Eckhardt, M. Kertesz, J. Chem. Phys. 1987, 87, 6687; (b) J.-M. Ducere, C. Lepetit, R. Chauvin, J. Phys. Chem. C 2013, 117, 21671; (c) X. Gao, H. Liu, D. Wang, J. Zhang, Chem. Soc. Rev. 2019, 48, 908.
[5] J. Arago, P. M. Viruela, E. Ortí, J. Mol. Struct. (THEOCHEM) 2009, 912, 27.
[6] I. Abdellah, V. Maraval, R. Chauvin, A. Poater, work in progress.
[7] See fror example: (a) K. Cocq, N. Saffon-Merceron, Y. Coppel, C. Poidevin, V. Maraval, R. Chauvin, Angew. Chem. Int. Ed. 2016, 55, 15133; (b) C. Zhu, A. Poater, C. Duhayon, B. Kauffmann, A. Saquet, V. Maraval, R. Chauvin, Angew. Chem. Int. Ed. 2018, 57, 5640; (c) C. Zhu, A. Poater, C. Duhayon, B. Kauffmann, A. Saquet, V. Maraval, R. Chauvin, Chem. Eur. J. 2021, 27, 9286.
Areas of expertise:
Acetylenic and carbo-mer chemistry
Carbon- and pi-electron-rich macro-cycles
Heterographdiyne
Expanded hereographene-like materials
Molecular fragments of carbon-based 2D materials
Required skills for the internship:
The student should be interested in the synthesis of molecular precursors and fragments of two-dimensional materials. He/she will be involved in organic synthesis, purification and characterization of molecules, and molecular fragments of thiographdiyne, and will participate to the discussions about the theoretical calculations that will be performed in parallel in Girona by Albert Poater on the targeted thiographdiyne 2D material.
He/she should have skills in synthesis, and in the classical methods of purification and characterization of organic molecules (NMR, MS, IR...).
